Date:2014-06-05

On May 20th, SINOMED released its upcoming European First-In-Man trial activity during an investigator kick-off meeting in collaboration with Cardialysis, the leading CRO company in Europe. This FIM trial is a prospective, multicenter, randomized study of BuMA Supreme™ DES comparing with Resolute Integrity in reducing restenosis in patients with de novo coronary artery disease. This generation is an improvement of stent material (Cobalt Chromium) based on the first generation (316L Stainless steel) where the rest remain the same. The primary endpoint in this study is in-stent late lumen loss (LLL) at 9 months after stent implantation assessed by off-line QCA with a sample size of 2×68 patients. The study will include four countries where 14 hospitals in Spain, Portugal, the Netherlands and Belgium will participate. In this trial, Prof. Patrick Serruys is positioned as chairman. Furthermore, Prof. Manel Sabaté (Clinic University Hospital Barcelona, Spain) and Prof. Clemens von Birgelen (Thorax Centrum Twente, the Netherlands) are positioned as Co-PI. The expected starting period will be in the fourth quarter of this year. Hence, with the advanced base coating technology, improvement of stent material, SINOMED anticipate to achieve promising results on safety and efficacy.
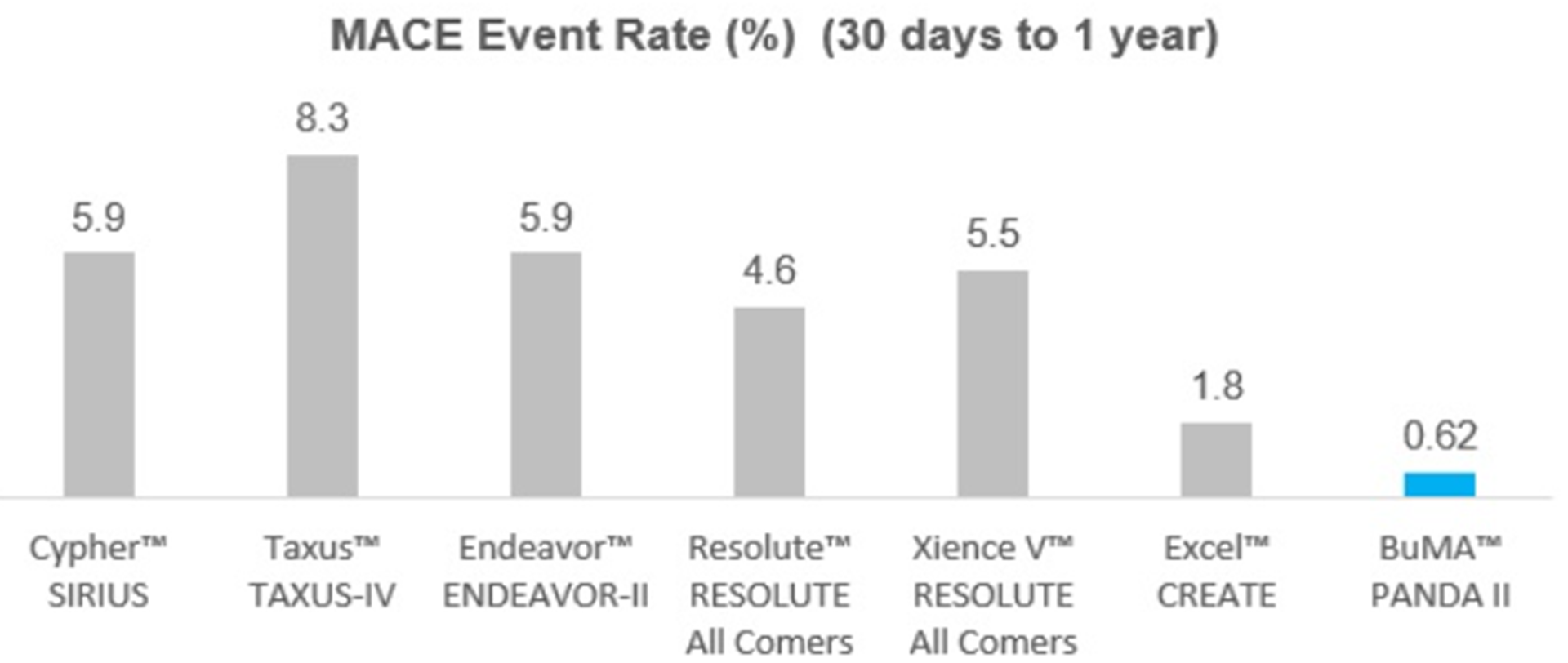
Furthermore, Jianhua Sun (CEO) provided an intriguing presentation on the core technology behind the product-line. Also, he revealed the latest outcomes of PANDA –II, a registry trial in China with circa 2415 patients registered in 50 centers. From 30 days after stent implantation to one year, MACE event rate is 0.62% (15 patients). As it is shown in the graph below, the MACE event rate of this trial is relatively low comparing to other stent studies. (See below PANDA II trial outcome)
Finally, SINOMED provided the attendants more information on its ongoing R&D of trans-catheter mitral valve replacement. The participants during the meeting were intrigued by the progress. More information will be revealed by the beginning of next year.