Drug Coated Coronary Stent System
HT Supreme®
The Healing-Targeted Drug Eluting Stent is designed specifically to allow natural healing through elution kinetics, biodegradable polymer and specialized coating. This new class of DES focused on rapid healing allows a quicker return of the protective endothelial layer.
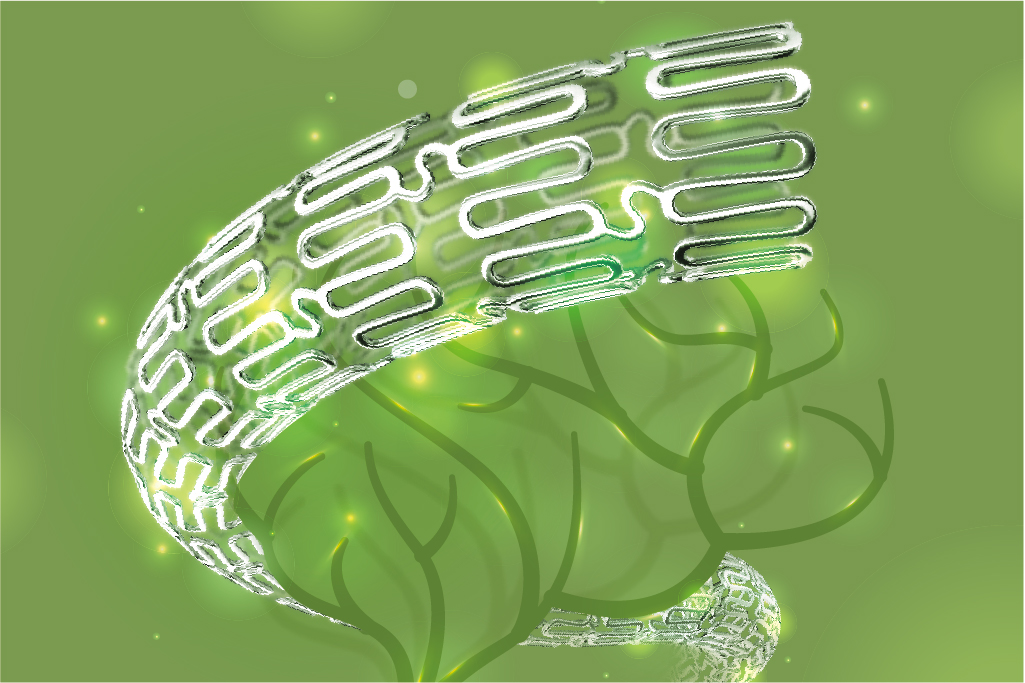
Intracranial Drug-eluting Stent System
NOVA DES™
NOVA DES™ is the world’s first drug-eluding stent as well as the first healing-targeted stent devoted to neuro-interventional treatment. Its unique drug coating design allows for rapid functional healing of the endothelial layer, reducing the risk of restenosis and stroke.
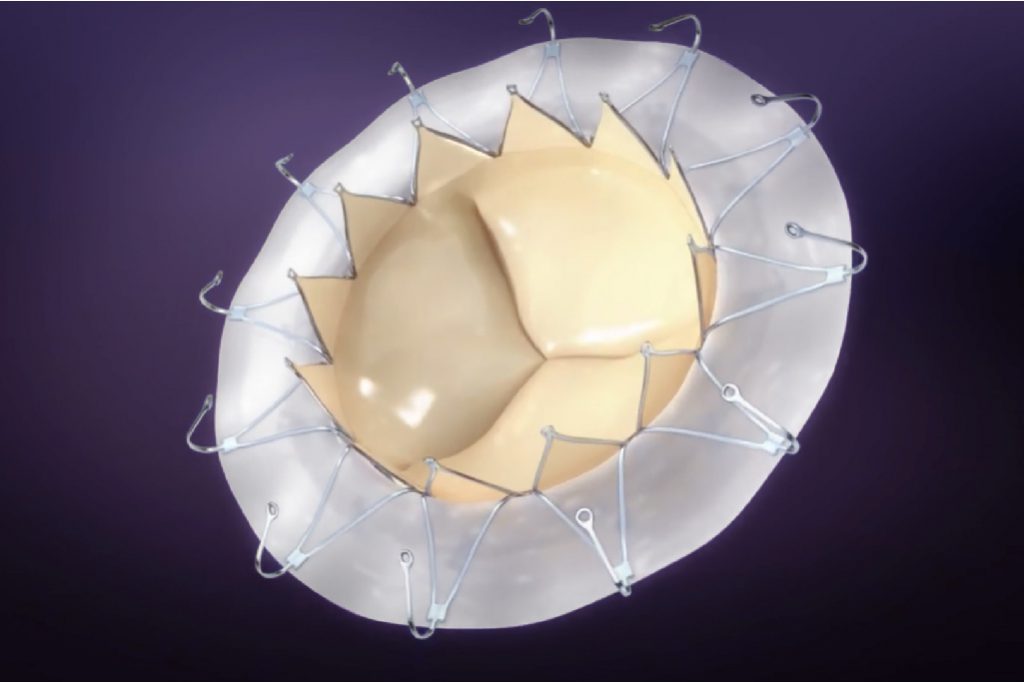
Transapical Mitral Valve Replacement System
AccuFit®
With unique self-expanding valve body design for optimal native valve “clipping” effect, and unique sealing ring design to minimize paravalvular leakage, AccuFit® is designed to address the challenges confronting minimally-invasive mitral valve replacement, and has gained wide attention in TMVR community worldwide.
WHO IS SINOMED
SINOMED is a global company engaged in patient-focused medical innovations for interventional medicine. We offer pioneering solutions for treating coronary, neurovascular and structural heart disease. We strive to combine the ingenuity of people with the power of technology to achieve new victories against cardiovascular disease.
The company was founded in 2007 as a startup company searching for a way to improve the common coronary stent. Since its humble beginnings, SINOMED has worked closely with industry thought-leaders expanding its product offering. Today, SINOMED is a publicly traded company on the Shanghai STAR market (Stock Code: 688108.SH) and one of the leaders in the Chinese coronary stent market.
0+
Products Used
0+
Clinical Study Samples
0%
Revenue Invested in R&D
0+
Employees Worldwide